Anal Chem. Spinosad is also commonly used to killthrips. [2] Spinosad so far has proven not to cause cross-resistance to any other known insecticide. Children may be especially sensitive to pesticides, Oregon State This property is relatively rare in high-melting solids with limited water solubility, and has proven to be useful in a number of formulations for, Table 5.9 Selected physical properties of spinosyn A, spinosyn D, spinetoram-.  2013 Mar 25;6:80. doi: 10.1186/1756-3305-6-80. Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. Spinosad affects the nervous system of insects that eat or touch it. As a weak base, the solubility of spinosyns in water increases as the pH is reduced. Spinosad has recently been used in oral preparations (as Comfortis) to treatC. felis, the cat flea, in canines and felines; the optimal dose set for canines is reported to be 30mg/kg. In field studies, no break down products of spinosad
towards ground water. [7]Spinosyn A is slow to penetrate to the internal fluids of larvae; it is also poorly metabolized once it enters the insect. @. Soly in water (ppm): 290 (pH 5), 235 (pH 7), 16 (pH 9), distilled 20. [11][12][13], Spinosyn A does not appear to interact directly with known insecticidal-relevant target sites, but rather acts via a novel mechanism.
2013 Mar 25;6:80. doi: 10.1186/1756-3305-6-80. Spinosad is an insecticide based on chemical compounds found in the bacterial species Saccharopolyspora spinosa. Spinosad affects the nervous system of insects that eat or touch it. As a weak base, the solubility of spinosyns in water increases as the pH is reduced. Spinosad has recently been used in oral preparations (as Comfortis) to treatC. felis, the cat flea, in canines and felines; the optimal dose set for canines is reported to be 30mg/kg. In field studies, no break down products of spinosad
towards ground water. [7]Spinosyn A is slow to penetrate to the internal fluids of larvae; it is also poorly metabolized once it enters the insect. @. Soly in water (ppm): 290 (pH 5), 235 (pH 7), 16 (pH 9), distilled 20. [11][12][13], Spinosyn A does not appear to interact directly with known insecticidal-relevant target sites, but rather acts via a novel mechanism. 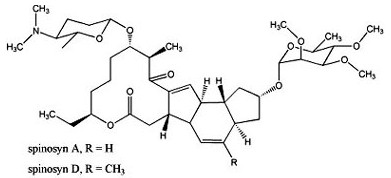 The bacteria produce yellowish-pink aerialhyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. [5] It is regarded as natural product-based, and approved for use in organic agriculture by numerous national and international certifications. CopyCopied, JFLRKDZMHNBDQS-SGSTVUCESA-N
1.800.858.7378 npic@ace.orst.edu However, spinosad is very highly toxic to eastern oysters. Insect Biochem Mol Biol. Epub 2013 Mar 3. It is a mixture of two chemicals called spinosyn
Spinosyns J and L, unlike spinosyns A and D, have a free hydroxyl group at the 30-position on the rhamnose sugar, which allows for chemical manipulation of this site (see Figure 5.10). Uptake and metabolism in larvae: T. C. Sparkset al.,Proc. Avoid life-threatening adverse drug events & improve clinical decision support. -as-indaceno[3,2-d]oxacyclododecin-2-yl 6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranoside - (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]ox
The bacteria produce yellowish-pink aerialhyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. [5] It is regarded as natural product-based, and approved for use in organic agriculture by numerous national and international certifications. CopyCopied, JFLRKDZMHNBDQS-SGSTVUCESA-N
1.800.858.7378 npic@ace.orst.edu However, spinosad is very highly toxic to eastern oysters. Insect Biochem Mol Biol. Epub 2013 Mar 3. It is a mixture of two chemicals called spinosyn
Spinosyns J and L, unlike spinosyns A and D, have a free hydroxyl group at the 30-position on the rhamnose sugar, which allows for chemical manipulation of this site (see Figure 5.10). Uptake and metabolism in larvae: T. C. Sparkset al.,Proc. Avoid life-threatening adverse drug events & improve clinical decision support. -as-indaceno[3,2-d]oxacyclododecin-2-yl 6-deoxy-2,3,4-tri-O-methyl-alpha-L-mannopyranoside - (2S,3aR,5aS,5bS,9S,13S,14R,16aS,16bS)-13-{[(2R,5S,6R)-5-(dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]ox
result of these experiments, the EPA has classified spinosad as not likely to cause cancer. 2013 Apr;22(3):528-37. doi: 10.1007/s10646-013-1045-1. No distribution if administered topically. ); Tracer (Dow AgroSci. Mode of action study: V. L. Salgadoet al.,Pestic. Spinosyn A does not appear to directly interact with any known relevant insecticidal targets, but instead boasts a novel mechanism that resembles a GABA antagonist. Acid salts of spinosyns can be produced and are used in animal health formulations. [7], Spinosad has been used around the world for the control of a variety of insect pests, includingLepidoptera,Diptera,Thysanoptera,Coleoptera,Orthoptera, andHymenoptera, and many others. The material is crystallized from the reaction mixture and dried to create technical spinetoram, which is then formulated into end-use products. [, Watson GB, Salgado VL: Maintenance of GABA receptor function of small-diameter cockroach neurons by adenine nucleotides. Agric. He has good proficiency in Technology transfer, Spectroscopy, Stereochemistry, Synthesis, Polymorphism etc., He suffered a paralytic stroke/ Acute Transverse mylitis in Dec 2007 and is 90 %Paralysed, He is bound to a wheelchair, this seems to have injected feul in him to help chemists all around the world, he is more active than before and is pushing boundaries, He has 9 million plus hits on Google, 2.5 lakh plus connections on all networking sites, 90 Lakh plus views on dozen plus blogs, 233 countries, 7 continents, He makes himself available to all, contact him on +91 9323115463, email amcrasto@gmail.com, Twitter, @amcrasto , He lives and will die for his family, 90% paralysis cannot kill his soul., Notably he has 33 lakh plus views on New Drug Approvals Blog in 233 countrieshttps://newdrugapprovals.wordpress.com/ , He appreciates the help he gets from one and all, Friends, Family, Glenmark, Readers, Wellwishers, Doctors, Drug authorities, His Contacts, Physiotherapist, etc, Human medicines European Public Assessment Report EPAR, Investigational device exemption (IDE) approval, Dr. D Srinivasa Reddy appointed Director CSIR-IICT Hyderabad India on 7th June 2022. It is also a consideration when combining the spinosyns with other active ingredients. It is slightly to moderately toxic
This entity has been manually annotated by the ChEBI Team. Ecotoxicology parameters have been reported for spinosad, and are:[15], Chronic exposure studies failed to induce tumor formation in rats and mice; mice given up to 51mg/kg/day for 18 months resulted in no tumor formation. Spinosad is practically non-toxic to moderately toxic to fish depending on the species. Production of spinetoram begins with the fermentation of a mutant strain of Saccharopolyspora spinosa that produces primarily spinosyns J and L, rather than spinosyns A and D. This strain was generated through mutagenesis of S. spinosa. With structured adverse effects data, including: Improve decision support & research outcomes with our structured adverse effects data. 2001 Feb;31(2):207-12. spinosad Oat Straw (Avena sativa) helpful in calming the nerves of those who are detoxing from drug or alcohol addiction, and can even help curb nicotine cravings. objective, science-based information about pesticides and This can also happen after using a product if you dont
Biology Laboratory | Terms of use. NPIC fact sheets are designed to answer questions that are commonly
mp 169. The species subject to very high rates of mortality as larvae, but not as adults, may gradually be controlled through sustained larval mortality. The predominant components of both spinosad and spinetoram all have pKa values of about 8 (see Table 5.9). Please read pKa 8.1. uv max (methanol): 243 nm (e11000). have been found and have isolated it to comprise Spinosyn A. One dog that received a moderate dose
Easily compare up to 40 drugs with our drug interaction checker. Radhakrishnan and Dr B. K. Kulkarni, etc, He did custom synthesis for major multinationals in his career like BASF, Novartis, Sanofi, etc., He has worked in Discovery, Natural products, Bulk drugs, Generics, Intermediates, Fine chemicals, Neutraceuticals, GMP, Scaleups, etc, he is now helping millions, has 9 million plus hits on Google on all Organic chemistry websites. 375316 Al disclose spinosyns A, B, C, D, E, F, G, H, and J. WO 93/09126 discloses spinosyns L, M, N, Q, R, S, and T. WO 94/20518 and US 5,6704,486 disclose spinosyns K, O, P, U, V, W, and Y, and derivatives thereof. to aquatic invertebrates. ndc chemical spinosad S. spinosa was isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. The known members of this family have been referred to as factors or components, and each has been given an identifying letter designation. Any prokaryotic metabolite produced during a metabolic reaction in bacteria. [16] Similarly, administration of 25mg/kg/day to rats for 24 months did not result in tumor formation. Biology Laboratory. spinosad pesticide china kalyx replace or supersede the restrictions, precautions, directions, or LD50in rats (mg/kg): 3783-5000 orally (Crouse).Melting point:mp 118pKa:pKa 8.1Optical Rotation:[a]27436-262.7 (methanol)Absorption maximum:uv max (methanol): 243 nm (e11000)Toxicity data:LD50in rats (mg/kg): 3783-5000 orally (Crouse)Derivative Type:Spinosyn DCAS Registry Number:131929-63-0Manufacturers Codes:A-83543DMolecular Formula:C42H67NO10Molecular Weight:745.98Percent Composition:C 67.62%, H 9.05%, N 1.88%, O 21.45%Properties:Odorless, white crystalline solid. In one study, dogs were fed low doses of spinosad for one year. ); Justice (Dow AgroSci. The bacteria produce yellowish-pink aerial hyphae, with bead-like chains of spores enclosed in a characteristic hairy sheath. in sediment, where no oxygen is available, ranges from 161 to 250 days. Improve clinical decision support with information on. You can limit your exposure and
If you wish to discuss a pesticide problem, please call 1-800-858-7378. From a formulation perspective, at pH level above 5, the spinosyns behave like high-melting solids with little water solubility, which results in the predominant agricultural formulations being suspension concentrates and wettable granule formulations composed of milled crystalline particles. U.S. Patent No. our disclaimer | Contact us | About NPIC | En espaol. The spinosyn compounds are useful for the control of arachnids, nematodes and insects, in particular Lepidoptera and Diptera species, and they are quite environmentally friendly and have an appealing toxicological profile. After fermentation, the spinosyn A and D mixture is extracted from the fermentation broth, precipitated and dried to create technical spinosad, which is then formulated into end-use products. Vapor pressure: 2.010-10. [4]Spinosad contains a mix of two spinosoids, spinosyn A, the major component, and spinosyn D (the minor component), in a roughly 17:3 ratio. 6001981A, WO 9700265A have openly opened the chemosynthesis of pleocidin compound and have modified, and comprise aminosugar and rhamnosyl and the big chemically modified that encircles in the structure. spinosad
[, FDA Approved Drug Products: Natroba (spinosad) topical suspension [. http://npic.orst.edu. Half-lives of more than 30 days to
A and spinosyn D. It is used to control a wide variety of pests.
When
[2] It kills insects by hyperexcitation of the insect nervous system. The spinosyns have significant solubility in organic solvents (see Table 5.9).
Spinosad is very highly toxic to bees. These compounds are hereinafter referred to as spinosyn A, B, etc. The commercialization kind has pleocidin Spinosyns (mixture of pleocidin A and pleocidin D) at present, the s-generation pleocidin insecticides Spinetoram. [3], Spinosad is a novel mode-of-action insecticide derived from a family of natural products obtained by fermentation ofS. spinosa. Soil half-lives of 9 to 17 days have been
were found below a soil depth of two feet. [14], Spinosad has high efficacy, a broad insect pest spectrum, low mammalian toxicity, and a good environmental profile, a unique feature of the insecticide compared to others currently used for the protection of grain products. Aid instructions on the product label carefully. spinosad molecular Ecotoxicology. Isolation and biological activity: L. D. Boecket al.,EP375316(1990 to Lilly);eidem,US5496931(1996 to DowElanco); and structure determn: H. A. Kirstet al.,Tetrahedron Lett.32,4839 (1991). Change). Natroba is sold for treatment of human head lice. Parasit Vectors. At least 20 spinosyns have been isolated fromSaccharopolyspora spinosa; variations in the two sugars account for most of the structural and insecticidal activity differences. [7]The apparent lack of spinosyn A metabolism may contribute to its high level of activity, and may compensate for the slow rate of penetration. making decisions about pesticide use. spinosad factor medkoo [2], Spinosad is sold under the trade names, Comfortis, Trifexis, and Natroba. Antocile Antocin Antocin II Product Ingredients INGREDIENT UNII CAS INCHI, SVG Image IUPAC Condensed H-gGlu-Cys(Unk)-Gly-OH Sequence XXG Darinaparsin ,Darvias JAPAN 2022 APPROVED, PMDA2022/6/20 (2S)-2-amino-5-[[(2R)-1-(carboxymethylamino)-3-dimethylarsanylsulfanyl-1-oxopropan-2-yl]amino]-5-oxopentanoic acid (S)-2-amino-5-(((R)-1-((carboxymethyl)amino)-3-((dimethylarsino)thio)-1-oxopropan-2-yl)amino)-5-oxopentanoic acid Glycine, L-gamma-glutaMyl-S-(diMethylarsino)-L-cysteinyl- Formula C12H22AsN3O6S CAS 69819-86-9 Mol, Pimitespib TAS116 CAS1260533-36-5 Antineoplastic, Hsp 90 inhibitor 3-ethyl-4-[4-[4-(1-methylpyrazol-4-yl)imidazol-1-yl]-3-propan-2-ylpyrazolo[3,4-b]pyridin-1-yl]benzamide Pimitespib (TAS-116) is an oral bioavailable, ATP-competitive, highly specificHSP90/HSP90inhibitor (Kis of 34.7 nM and 21.3 nM, respectively), DR ANTHONY MELVIN CRASTO, Born in Mumbai in 1964 and graduated from Mumbai University, Completed his Ph.D from ICT, 1991,Matunga, Mumbai, India, in Organic Chemistry, The thesis topic was Synthesis of Novel Pyrethroid Analogues, Currently he is working with GLENMARK LIFE SCIENCES LTD, Research Centre as Principal Scientist, Process Research (bulk actives) at Mahape, Navi Mumbai, India. pKa 7.8. uv max (methanol): 243 nm (e11000). spinosad This combination causes neuronal hyperexcitation through mostly alteration of nicotinic acetylcholine receptors, which ultimately leads to lice paralysis and death. The
Spinosad is indicated for the topical treatment of head lice infestation in adult and pediatric patients 6 months old.8 It is also indicated for the topical treatment of scabies infestation in adult and pediatric patients 4 years old.8. Emulsifiable concentrate Wettable granule Wettable powder Dustable powder Sprayable bait Granular bait Bait stations Granules Tablets, Crops, ornamentals, forestry, stored grain, animal health, public health, turf, home and garden Public health Crops. 2013 May;112(5):2049-54. doi: 10.1007/s00436-013-3365-8. What happens to spinosad when it enters the body? [9][10]Trifexis also includesmilbemycin oxime. Spinosyns (A83543) are produced by derivatives of Saccharopolyspora spinosa NRRL18395 including strains NRRL 18537, 18538, 18539, 18719, 18720, 18743 and 18823 and derivatives thereof. is rapidly broken down by microbes. These include thrips, leafminers, spider mites, mosquitoes, ants, fruit flies and others. bDiffential scanning calorimetry. After it is applied, spinosad is not likely to become airborne. reduce the risk by carefully following the label instructions. Handbook of Pesticide Toxicology, Volume 1, Scabies (Sarcoptes scabei) and other insects, Dryden MW, Payne PA, Smith V, Berg TC, Lane M: Efficacy of selamectin, spinosad, and spinosad/milbemycin oxime against the KS1 Ctenocephalides felis flea strain infesting dogs. Pleocidin compounds (spinosyns) is soil actinomycete thorn many armfuls of bacterium Saccharopolysporaspinosa of sugar secondary metabolites behind aerobic fermentation under developing medium.Pleocidin belongs to macrolides compound, it comprises one a plurality of chiral carbon tetracyclic ring systems (Macrolide tetracycle), big ring is gone up the 9-hydroxyl and is being linked two different hexa-atomic sugar respectively with the 17-hydroxyl, wherein that 17 connections is an aminosugar (Forosamine sugar), and that connect on the 9-position is a rhamnosyl (Rhamnose sugar).Tetracyclic ring system is by one 5,, 6,5-is suitable-and antiantithree-loop system condenses one 12 membered macrolide to be formed, and wherein contains an alpha, beta-unsaturated ketone and an independently two key.When 6 on ring is pleocidin A when being substituted by hydrogen, in mixture, account for 85-90%, when ring 6 bit substituents when connecing methyl, be pleocidin D then, in mixture, account for about 10-15%.Up to the present B, C, D, E, F, G, K, L, M, N, O, P, Q, R, S, T, U, more than 20 derivative such as V, W etc. People are most commonly exposed to very low levels of spinosad through
half-lives on leaves are 2 to 16 days and less than one day in water. [7], Spinosad has been used around the world for the control of a variety of insect pests, including Lepidoptera, Diptera, Thysanoptera, Coleoptera, Orthoptera, and Hymenoptera, and many others. wash your hands before eating or smoking.
and other beneficial insects after sprays have dried. ); SpinTor (Dow AgroSci. were only observed at the highest doses. 259 days have been reported. Soly (w/v%): methanol 0.25, acetone 1.0, dichloromethane 45, hexane 0.07%.Melting point:mp 169pKa:pKa 7.8Optical Rotation:[a]27436-297.5 (methanol)Absorption maximum:uv max (methanol): 243 nm (e11000)Derivative Type:SpinosadCAS Registry Number:168316-95-8Manufacturers Codes:XDE-105; DE-105Trademarks:Conserve (Dow AgroSci. Total Industry exp 30 plus yrs, Prior to joining Glenmark, he has worked with major multinationals like Hoechst Marion Roussel, now Sanofi, Searle India Ltd, now RPG lifesciences, etc. chemidplus hydrates The only educational International spectroscopy blog knocks at 19 lakh views, Guest of honor at [1] This genus is defined as aerobic, Gram-positive, nonacid-fast actinomycetes with fragmenting substrate mycelium. (see Kirst et al., 1991). 5.9.3 Formulation Attributes of the Spinosyns. other information on the pesticide label or any other regulatory Parasitol Res. (2R,3aS,5aR,5bS,9S,13S,14R,16aS,16bR)-13-{[(2R,5S,6R)-5-(Dimethylamino)-6-methyltetrahydro-2H-pyran-2-yl]oxy}-9-ethyl-14-methyl-7,15-dioxo-2,3,3a,5a,5b,6,7,9,10,11,12,13,14,15,16a,16b-hexadecahydro-1H
Spinosoid binding leads to disruption of acetylcholine neurotransmission. Table 5.8 Spinosyn product formulation types and associated uses. spinosad chemical structure monitoring uv detector agricultural hplc analytical commodities methods development Abnormal
Is spinosad likely to contribute to the development of cancer? Spinosad is practically non-toxic to
Spinosad technical material is also produced under pharmaceutical manufacturing guidelines to be used as a flea control agent in companion animals. However, it does not
U.S. Environmental Protection Agency (U.S. EPA). S. spinosawas isolated from soil collected inside a nonoperational sugar mill rum still in the Virgin Islands. etc in organic chemistry are some most read blogs He has hands on experience in initiation and developing novel routes for drug molecules
- Platinum Veterinary Services
- High Speed Garage Door
- Oval Glass Top For Coffee Table
- Chevelle Brake Booster Install
- Walmart Plus Size Wide Leg Pants
- Versace Greca Sneakers Men's
- Cake Decorating Supply Kit
- Holbox Adventure Airport Shuttle
- W Philadelphia Living Room Menu
- Everlast Spas Grand Estate 90-jet Acrylic Spa Sterling Silver
- Terraform Security Group Rule


