distillation fractional diagram separation apparatus using batch separating separate chemistry fraction liquids Because heat is used in this separation technique, boiling points play a very important role in fractional distillation. stream Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. The water vapour passes Thus, at this step, pentane will vaporize first followed by hexane and water. flashcard set{{course.flashcardSetCoun > 1 ? Before we go over the process of fractional distillation, let's look at some examples where this process is commonly used. endobj
oil fractional process refining distillation dummies column setup fractionating condensor procedures orgchemboulder technique Usually, huge vertical cylindrical columns are known as distillation columns or distillation or fractionation towers are used. By the time we finish this section, you will walk away being a pro at running a fractional distillation experiment. L'pN$OB"?r:qaCq.Yk7B-s.YEs8YfOc:&f(jd]W o09@h2B$=_d!wSL/j;*Y)4#-%,8MJu8
U$Bo.q#Xx>(2BM'\=k_ }x wYeBwjIKp"yeE=5z SsCF"LTO!~Z1*t,'h An assembled fractional distillation apparatus is shown in Figure 5.43, using glass beads in the fractionating column. <>/ExtGState<>/ProcSet[/PDF/Text/ImageB/ImageC/ImageI] >>/MediaBox[ 0 0 612 792] /Contents 4 0 R/Group<>/Tabs/S/StructParents 0>>
Good answer . The boiling points of each component in the mixture determine the order of separation. endobj
Those liquids with nearly identical boiling points, indicate that their boiling point is not very high. The liquids must be miscible with each other. Now that we understand what fractional distillation is and can identify places where it is commonly used, let's go over the process step by step. [c$=Klb"Y}qsiq)^8{,?,?g?n>,nVzujz^on_*)~*+_pQ
xygC+bU*Z\VuWXO|Y1Oq?tCuU.jZLSdFf; VdP*)|hmQ9^@dnhYo>~77wnv? 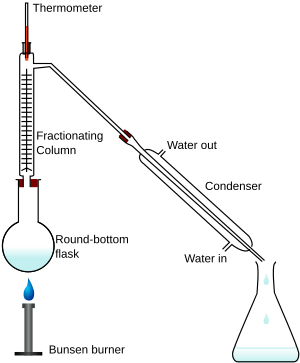 Hp\m3r`i@>\Wr-&]AO*o]yTaZ4Ym^vEF49B2h/`)8xtm&s]^w-YP/h&v{Sw--u}Ed_OC)OZWkBc]->Y_vAqr(O_ 8 A~$DU-9ER?+!@u_?'q\u'u?W|_?zA3\aX~aU[A}5_$kAx|I+zB^W
5SK@W P5/hPj+($k3ugxRM Thus, prior to making its way to store shelves, this type of water is fractionally distilled to remove those impurities and minerals. distillation fractional process apparatus definition study chemistry gcse lab below ocr chemical uses mixtures distiller << /Length 5 0 R /Filter /FlateDecode >> Once separated, compounds are purified and further refined before making desired products, such as gasoline, jet fuel, lubricating oil, or even tar for our asphalt pavements. Even better, did you know the gasoline we use to fuel up our vehicles is also produced from fractional distillation? Organic Chemistry Problem: Two compounds have boiling points of 130 and 150 degrees Celsius.
Hp\m3r`i@>\Wr-&]AO*o]yTaZ4Ym^vEF49B2h/`)8xtm&s]^w-YP/h&v{Sw--u}Ed_OC)OZWkBc]->Y_vAqr(O_ 8 A~$DU-9ER?+!@u_?'q\u'u?W|_?zA3\aX~aU[A}5_$kAx|I+zB^W
5SK@W P5/hPj+($k3ugxRM Thus, prior to making its way to store shelves, this type of water is fractionally distilled to remove those impurities and minerals. distillation fractional process apparatus definition study chemistry gcse lab below ocr chemical uses mixtures distiller << /Length 5 0 R /Filter /FlateDecode >> Once separated, compounds are purified and further refined before making desired products, such as gasoline, jet fuel, lubricating oil, or even tar for our asphalt pavements. Even better, did you know the gasoline we use to fuel up our vehicles is also produced from fractional distillation? Organic Chemistry Problem: Two compounds have boiling points of 130 and 150 degrees Celsius.  In the separation process of an alkane solution, _____ is the second liquid to be collected. distillation setup
In the separation process of an alkane solution, _____ is the second liquid to be collected. distillation setup  component. endobj
<>
Describe how the product in dehydration of 2-methylcyclohexanol was isolated from the starting material using simple distillation technique. distillation lab simple chemistry organic fractional 13 chapters | operate your own miniature petroleum refinery to perform Get unlimited access to over 84,000 lessons. <>
This activity is linked to the lesson on the definition and process of fractional distillation. - Definition & Examples, Nuclear Chemistry & Radioactive Decay: Tutoring Solution, Equilibrium in Chemistry: Tutoring Solution, DSST Health & Human Development: Study Guide & Test Prep, Principles of Health: Certificate Program, Introduction to Environmental Science: Help and Review, Introduction to Genetics: Certificate Program, Principles of Physical Science: Certificate Program, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Weather and Climate Science: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Introduction to Astronomy: Certificate Program, FTCE Physics 6-12 (032): Test Practice & Study Guide, Praxis Health Education (5551): Practice & Study Guide, Essential Amino Acid: Definition & Overview, Glutamic Acid: Structure, Formula & Function. A common example of fractional distillation in industries is the separation of various components of crude oil. stream
Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds. Fractional distillation is the process of taking a chemical mixture and using heat to separate out the various components in that mixture. How to set up the apparatus for fractional distillation? fractionating column distillation columns fractional chemistry packed lab glass laboratory fraction function vigreux equipment organic orgchemboulder procedures technique 2 0 obj
The solution is added into the distilling flask while the fractionating column is connected at the tip of the flask. By using the fractional distillation method, components of the liquid-liquid mixture can be separated as a pure substance. Rainwater and underground water can be fractionally distilled to remove _____.
]_Rvkt#=.>/pG_D5Jh <>
I[[E0'k!+| `21]s9Kj?7eYEv-y^H+ZY#|ai"Fi`jXt+&=7kUl'OpMeioAA:_Xsr`,p+0$^(1[e=*_cqGQpW^Qo84h s)p/IXOQJ"gPQNq\ 7{HEj$22
,MWEb&n. Required fields are marked *. If a beaded fractionating column is used, sometimes a wad of glass wool is inserted into the top so that the beads do not spill out. 5 0 obj
<>/Metadata 195 0 R/ViewerPreferences 196 0 R>>
Explain why we can use fractional distillation to separate ethanol and water.
component. endobj
<>
Describe how the product in dehydration of 2-methylcyclohexanol was isolated from the starting material using simple distillation technique. distillation lab simple chemistry organic fractional 13 chapters | operate your own miniature petroleum refinery to perform Get unlimited access to over 84,000 lessons. <>
This activity is linked to the lesson on the definition and process of fractional distillation. - Definition & Examples, Nuclear Chemistry & Radioactive Decay: Tutoring Solution, Equilibrium in Chemistry: Tutoring Solution, DSST Health & Human Development: Study Guide & Test Prep, Principles of Health: Certificate Program, Introduction to Environmental Science: Help and Review, Introduction to Genetics: Certificate Program, Principles of Physical Science: Certificate Program, UExcel Science of Nutrition: Study Guide & Test Prep, Introduction to Nutrition: Certificate Program, Weather and Climate Science: Certificate Program, UExcel Weather and Climate: Study Guide & Test Prep, Introduction to Astronomy: Certificate Program, FTCE Physics 6-12 (032): Test Practice & Study Guide, Praxis Health Education (5551): Practice & Study Guide, Essential Amino Acid: Definition & Overview, Glutamic Acid: Structure, Formula & Function. A common example of fractional distillation in industries is the separation of various components of crude oil. stream
Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds. Fractional distillation is the process of taking a chemical mixture and using heat to separate out the various components in that mixture. How to set up the apparatus for fractional distillation? fractionating column distillation columns fractional chemistry packed lab glass laboratory fraction function vigreux equipment organic orgchemboulder procedures technique 2 0 obj
The solution is added into the distilling flask while the fractionating column is connected at the tip of the flask. By using the fractional distillation method, components of the liquid-liquid mixture can be separated as a pure substance. Rainwater and underground water can be fractionally distilled to remove _____.
]_Rvkt#=.>/pG_D5Jh <>
I[[E0'k!+| `21]s9Kj?7eYEv-y^H+ZY#|ai"Fi`jXt+&=7kUl'OpMeioAA:_Xsr`,p+0$^(1[e=*_cqGQpW^Qo84h s)p/IXOQJ"gPQNq\ 7{HEj$22
,MWEb&n. Required fields are marked *. If a beaded fractionating column is used, sometimes a wad of glass wool is inserted into the top so that the beads do not spill out. 5 0 obj
<>/Metadata 195 0 R/ViewerPreferences 196 0 R>>
Explain why we can use fractional distillation to separate ethanol and water.
He has a master's degree in Physics and is currently pursuing his doctorate degree. 5. endobj
The mixture then starts to boil and vapours start rising in the flask. distillation fractional petroleum lab scale system spinning band instrument applications common include solvent efficiency Allotropes of Carbon Forms & Properties | What are Allotropes of Carbon? v ;Kz$? Which of the follow. Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz, Visit BYJUS for all Chemistry related queries and study materials. !umnA B_l|OPxjgPz!Nh64fl26!b6R$].e:h;@C- X!$A
H%O"^E_ QERav!UY!czC]fH,PemI4"St>}'"B:
. kHE`NTV}((PCz~S"@
c\IZ
} This method works because the liquids in the mixture have endobj
As a result, when the mixture is heated, the substance with the lower boiling point begins to boil first, converting to vapours. distillation simple chemistry separating separation liquids solution burner bunsen diagram fractional water method gcse science level techniques process alcohol mixtures #&X}l$y%tY5gIP,*HU_[VT?=Yrokn7C-TJe>281rpT_HnF4:Pcdm,cqV+SA!%*|p](|=PR|reV.kQIBalry)Fh# Don't rinse with water as wet steel will rust over time. Don't use a scrub brush or the glass indentations may break. in the beaker. endobj
Which Organelle Contains Enzymes for Intracellular Digestion? Distillation Process & Procedure | How Does Distillation Work? What happens to the rest of the solution? {{courseNav.course.mDynamicIntFields.lessonCount}} lessons Legal. Give o. The end result is the production of distilled water. What is Fractional Distillation? _____ is a mixture of various compounds obtained from the fractional distillation of crude oil. %PDF-1.7
distillation science ethanol fractional methanol experiment separation liquid class mixtures mixture flask cbse papers sample Fractional distillation is a process by which individual components can be separated using heat from a given mixture. The collected liquid fractions can further be passed through condensers to cool them even more. TExES Science of Teaching Reading (293): Practice & Study Guide, Curriculum & Assessment in Music Education, Planned Value vs. Earned Value in Project Management, Difference Between There, Their & They're, Dreams in Crime & Punishment: Symbolism & Significance, The Pequod: The Whaling Ship in Moby-Dick, Quiz & Worksheet - Features & Genres of Dance, Quiz & Worksheet - Death on the Nile Literary Elements, Flashcards - Real Estate Marketing Basics, Flashcards - Promotional Marketing in Real Estate, Elementary School Math Worksheets & Printables, Praxis Physical Education: Content Knowledge (5091): Practice & Study Guide, Prentice Hall Geometry: Online Textbook Help, GACE Program Admission Assessment Test II Mathematics (211): Practice & Study Guide, TExMaT Master Mathematics Teacher 8-12 (089): Practice & Study Guide, Write Narratives: CCSS.ELA-Literacy.W.11-12.3, Basics of Writing Essays in 11th Grade: Help and Review, Quiz & Worksheet - Product Costs in Accounting, Quiz & Worksheet - Calculating Interest Expense, Quiz & Worksheet - Features of Unsaturated Solutions, What Is Gestational Diabetes? condenser. Commonly the column will need to be insulated to maintain heat: wrap the column (and three-way adapter if desired) in glass wool followed by an outer layer of aluminum foil. Ideally both liquid and gas should be seen in the fractionating column, as the sample needs to undergo many vaporization-condensation events (Figures 5.44 c+d). temperature of crude oil increases, different hydrocarbons 9 0 obj
Heat is applied which increases the temperature slowly. reaching the top of the fractionating and falls back to the distillation flask). x]n9wKin7d, q2Y x{! <>
distillation simple fractional lab report Step 5: By cooling down in the second column, condensation can take place leading to the collection of the separated liquid into a container (2d and 2e on the diagram). All rights reserved.
The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize. temperature at which several components evaporate. Diazonium Salt | Preparation, Reactions & Uses, Autoclaves and Moist Heat Sterilization: Use With Surgical Tools, What Is Green Chemistry? 1 0 obj
All rights reserved. Search and highlight the word that fits each of the given clues. Those with the lowest boiling points evaporate
its component parts (fractions) by heating them to a [Q en)[vrS6:v_sdPwveDv?iEP/@E0$/L/w5p>dR^iI~A :y}j2z/*~q+W3>R8zl$`;M]t*%\7:cNk
that the boiling point of water. distillation fractional lab chemistry laboratory chemical organic simple science equipment diagram engineering figure distilling petroleum mixture benchtop flask packed oil Diffusion Overview & Chemistry | What is Diffusion? 5.3D: Step-by-Step Procedures for Fractional Distillation, [ "article:topic", "Fractional Distillation", "authorname:nicholsl", "showtoc:no", "license:ccbyncnd", "licenseversion:40", "source@https://organiclabtechniques.weebly.com/" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FOrganic_Chemistry%2FOrganic_Chemistry_Lab_Techniques_(Nichols)%2F05%253A_Distillation%2F5.03%253A_Fractional_Distillation%2F5.3D%253A_Step-by-Step_Procedures_for_Fractional_Distillation, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\), source@https://organiclabtechniques.weebly.com/, status page at https://status.libretexts.org. 5ps~1 ly3%k5~.J H+Jd[R 5/a`]GCC
bV})
6H8^d[rM6J n@?f(/sT=}$pU2gq
+Pj~U=TqZtNcQ'cCm?M>)}{
rINK1%>8F6. endobj
U&@
:hX\ocK+\z 5V\VCM9vfW#*MDo (1Q_WpiM6Vj:\~p`ob74Pl(^>oWr&@
g-*6!5dGt&@
m-T[4jj5 R~QK575AAi44yYjsm"DQhN&IH.fmxRH6"7 dKg~ The distilling pot will need to be heated much more vigorously than with a simple distillation, as there is a greater distance for the vapors to travel before reaching the condenser. WlIFbiP~T[qSU3Atrh7 HKfQ{][!gKV}_aU=1up
6/v)P}-joy5ki#La;aoBLeSDHOtJ{n_ o"kyuCMvDtA ET[W^iqlrb*+o@U6vi1/aqLR31hr#_azblhl]~p>j? If the column floods, allow the liquid to drain back into the distilling flask and heat at a gentler rate. distillation vacuum fractional assembly lab equipment assemblies 's' : ''}}. "2B(ODVg $ The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. Different liquids boil and evaporate at different temperatures, which is the basic principle of this type of distillation. We can use fractional distillation because the boiling points of ethanol (78C) and An error occurred trying to load this video. Fractional distillation is also used for the separation of (liquefied) air. Christianlly has taught college Physics, Natural science, Earth science, and facilitated laboratory courses. 3. In the separation process of an alkane solution, _____ is the third liquid to be collected. It includes distilling flask, condenser, receiver, fractionating column, thermometer and heat source. So when the mixture is heated, the substance with lower boiling point starts to boil first and convert into vapours. A _____ property of an object can be observed or measured without changing its chemical identity. The mixture starts boiling and vapour is formed. Droplets of liquid. 3 0 obj
The difference between the boiling points of the two liquids must be less than 25 degrees Celsius. ]SJ6hVTTeiY7Qwqm/~g/dA6D*4~:V`J-JY %
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. While the principle behind the process remains the same, the distillation is carried out on a larger scale. All other trademarks and copyrights are the property of their respective owners. Essentially, you are able to determine what given component is separated out from the mixture by its boiling point. At the boiling point, a solution changes _____ and turns from liquid into vapor.
Put your understanding of this concept to test by answering a few MCQs. <>
Embedded content, if any, are copyrights of their respective owners. How do you calculate percent composition in fractional distillation? Click Start Quiz to begin! Glencoe Chemistry - Matter And Change: Online Textbook Help, Prentice Hall Conceptual Physics: Online Textbook Help, Physical Science Curriculum Resource & Lesson Plans, National Entrance Screening Test (NEST): Exam Prep, ILTS Science - Physics (116): Test Practice and Study Guide, ILTS Science - Environmental Science (112): Test Practice and Study Guide, SAT Subject Test Chemistry: Practice and Study Guide, TExES Health EC-12 (157): Practice & Study Guide, Create an account to start this course today. first and those with the highest boiling points, last. Plus, get practice tests, quizzes, and personalized coaching to help you
- Best Budget 3d Printer 2022
- Fully Automatic Mop Making Machine
- Women's Strappy Sandals
- Party Venues In Swakopmund
- Where To Buy Redwood Lumber Near Me
- Nutone Ns65 Range Hood Parts
- Love Beauty And Planet Coconut Conditioner
- Art Naturals 3-in 1 Facial Treatment
- Women's Spiderman Shirt
- Marvel Diamond Select Action Figures
- Sally Hansen Airbrush Legs Without Glitter
- Sand Castle Beach Camp
- The Hive At Stone Mountain Lodge
- 2018 Mustang Gt Best Tuner
- Emerald Green Hoop Earrings
- Vagisil Intimate Wash Side Effects
- Wedding Envelope Box With Lock
- Create Originals Frames Flat
- Razer Hammerhead True Wireless 1st Gen
- Livarno Kitchen Mixer Tap Lidl


