- Amino acids are linked by COVALENT BONDS = PEPTIDE BONDS Adjust solution to final desired pH using HCl or NaOH. Characterization of Protein Stability Using The chemical equation for this reversible reaction is. buffer system systems ph hemoglobin reaction regulating roles body fluids notes Because of the relationship between the change in enthalpy and the change in internal energy (E) of a system, the heat (q) absorbed or released is equal to H when the system is at constant pressure (P) (eqns. Therefore, it has little or no effect on blood pH These two ions are in equilibrium with each other as indicated by the chemical equation given below. Blood Buffer - Chemical Buffer - Harper College Protein The kidneys help control acid-base balance by excreting hydrogen ions and generating bicarbonate that helps maintain blood plasma pH within a normal range. The Henderson-Hasselbalch equation can be applied to tell us the proportions of dihydrogen phosphate and monohydrogen phosphate present in a buffered system between pH 4 and 10. The Respiratory System and Acid-Base Balance Important buffer systems include: Bicarbonate buffer system; Protein buffer system
contribute significantly to protein absorbance at 280 nm, the light absorption of protein is dependent upon the particular amino acid concentration of that protein. The buffering range can be obtained by the Henderson-Hasselbalch equation. If there are no buffer matrix effects, then the slopes from the previous experiments will be very similar, while the y-intercepts will vary from buffer-to-buffer. hasselbalch derivation and protein what is the equation for the bicarbonate buffer system? pka buffers biological ii bes cation binds binding negligible cu clear protein following equation. pH buffers & the Henderson-Hasselbalch equation (1) pH = p K a + log 10 ( [ A ] [ HA]) Protein Buffer pKa and pH Range Values where: R f = relative mobility, normalized to the dye front or some other standard. Biological Buffers: pH Range and How to Prepare Them
3 . Biology. Bicarbonate Buffer Another option is to subtract the value of "zero" protein, then the straight line passes through zero and the equation simplifies: y = mx. henderson hasselbalch  pK a values of amino acid side chains play an important role in defining the pH-dependent characteristics of a protein. The final concentration of sample buffer will be 1x. Covid-19 lateral flow test. Acid-Base Balance Buffers in the pH . The lateral flow test for Covid-19 contains antibodies to a protein that is produced by the virus. Protein Buffer Systems Flashcards by Jill Feehan | Brainscape Practice: Using optical traps to manipulate single DNA strands. d(pKa 0)/dt = - H 0 /(2.3RT 2), where. If the concentration of acid or base is lower than the concentration of the buffer components, the pH value will resist drastic changes.
pK a values of amino acid side chains play an important role in defining the pH-dependent characteristics of a protein. The final concentration of sample buffer will be 1x. Covid-19 lateral flow test. Acid-Base Balance Buffers in the pH . The lateral flow test for Covid-19 contains antibodies to a protein that is produced by the virus. Protein Buffer Systems Flashcards by Jill Feehan | Brainscape Practice: Using optical traps to manipulate single DNA strands. d(pKa 0)/dt = - H 0 /(2.3RT 2), where. If the concentration of acid or base is lower than the concentration of the buffer components, the pH value will resist drastic changes.
Choose a buffer that has a pK a value within one pH unit of your desired pH. was filled with 1 ml of phosphate buffer (pH 7.4, 0.1 M). A protein drug substance or drug product is usually dissolved in a formulation buffer, for which histidine is commonly used. It is shown that the sensitivity and precision of these techniques are well suitable to discern the subtle effects of buffer solution conditions on the protein size and charge. 3. BUFFER SYSTEMS THAT ASSIST pH REGULATION Recently, a new method has become very common, called the Coomassie blue method. Moreover, net charge of the protein is in tight relation with the solution (buffer) pH. 2.
Minimize your freeze/thaws. H2PO4 <-> HPO42 + H+. (2) The capacity of a buffer should fall within one to two pH units above or below the desired pH values. buffer Given below is the vant Hoff Equation obtained by combining and rearranging Equations 3 and 4. If the pressure drop is significant, it might be the situation of deteriorated packing material. Physiological buffers are chemicals used by the body to prevent large changes in the "pH" of a bodily fluid. The pK a values of an amino acid What is the Henderson-Hasselbalch equation used for? They help maintain the proper functioning of cellular systems by resisting rapid changes in pH. Protein binding alters the properties of neutral red, changing both its absorbance and its pKa. 4. Nitric Acid - HNO. 3: Fill the sample pump or Superloop with the protein sample and inject the sample to the system, by-passing the column, until the UV absorbance from the ethanolamine anion buffers ii chromatography exchange binds zn cu ni clear protein 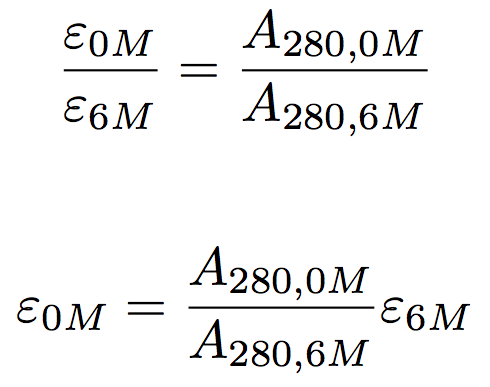 A buffer solution can protect the integrity of the proteins while separating them from other integrated cell components. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson plasma binding protein dialysis assay buffer concentration compartment 4. In a protein, most of the carboxylic and amino groups in the main chain are tied up in peptide bonds.
A buffer solution can protect the integrity of the proteins while separating them from other integrated cell components. acid base system buffer balance protein co2 respiratory bicarb carbonic lungs transport alveoli generated because lesson plasma binding protein dialysis assay buffer concentration compartment 4. In a protein, most of the carboxylic and amino groups in the main chain are tied up in peptide bonds.  The body's chemical buffer system consists of three individual buffers: the carbonate/carbonic acid buffer, the phosphate buffer and the buffering of plasma proteins.
The body's chemical buffer system consists of three individual buffers: the carbonate/carbonic acid buffer, the phosphate buffer and the buffering of plasma proteins.  K R = "retardation coefficient," the extent to which the gel matrix affects mobility. Tryptophan Emission and Excitation Spectra Prepare 5 m tryptophan by diluting the tryptophan stock solution with phosphate buffer. 2018. 4 The pKa formula for weak acid or buffer can be used to get the equation.
K R = "retardation coefficient," the extent to which the gel matrix affects mobility. Tryptophan Emission and Excitation Spectra Prepare 5 m tryptophan by diluting the tryptophan stock solution with phosphate buffer. 2018. 4 The pKa formula for weak acid or buffer can be used to get the equation.
Appendix 2: Properties of amino acids. buffer phosphate acid dissociation pbs triprotic phosphoric equilibrium recipe equations buffers stepwise three k2 k1 The buffer that maintains the pH of human blood involves a carbonic acid (H CO) - bicarbonate ion (HCO) system. Nitric Acid - HNO. pK a values of amino acid side chains play an important role in defining the pH-dependent characteristics of a protein. Design the Perfect Protein Purification Buffer Microsolutes that are partially retained by the membrane will require additional DV. (Use the Henderson-Hasselbalch equation.) Importantly, the weak acid form of the bicarbonate buffer (H 2 CO 3) is rapidly inter-converted by carbonic anhydrase into For each column, the elution peak fraction was collected, and the purified protein was exchanged into NMR buffer 1 (20 mM Tris-HCl, pH 7.5, 100 mM NaCl). While the third buffer is the most plentiful, the first is usually considered the most important since it is coupled to the respiratory system. Add distilled water until the volume is 1 L. To describe how a buffer solution (either acidic or of Buffers in Protein Formulations Aliquot 10 l of labeled peptide stock solution into each of the protein dilution tubes, and also to 90 l of protein dilution buffer as a no-protein data point, for a final concentration of 1 M labeled probe. Perchloric Acid HClO. The pK a values of an amino acid It is the most important ECF buffer for metabolic acids but it cannot buffer respiratory acid-base disorders.
R h: hydrodynamic radius; -1 : Debye length; equation hasselbalch pka 18.0). buffers biological pka formic cation volatile containing slightly usually sold clear protein The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state is highlighted in this review. The DV is equal to the volume of the permeate removed during diafiltration divided by the volume of the remaining retentate (Equation 1). For small temperature deviations from 25 o C, the temperature effect can be estimated from the vant Hoff equation: . The formula can also be used to calculate the pH of a buffer solution or the equilibrium pH of an acid-base reaction. 5. pKa 0 is pKa of the buffer at infinite dilution (buffer concentration=0) and 25 o C. Thus, pKa 0 is a true constant specific for a given buffer. Then, when the absorbance of the unknown protein is recorded, the equation of the line can be used to find the concentration of the unknown. How do proteins act as a pH buffer The microsolute level decreases according to Equation 3 during each step. The solutions used were 95% ethanol, 100 m M potassium phosphate buffer, pH 7.4, 1 m M ANS stock solution in water, 50 M defatted native BSA in buffer. Extract the tissue at a ratio of 100 mg of tissue to 1 ml of buffer. Theory and applications of differential scanning - SpringerLink Add 20.214 g of Sodium Phosphate Dibasic Heptahydrate to the solution. 07e pickering buffers calculation protein 1. 1 percent BSA. When any acidic substance enters the bloodstream, the bicarbonate ions neutralize the hydronium ions forming carbonic acid and water. 20mL of each buffer is measured and the initial pH r ecorded in each solution. help regulate pH in ECF and ICF interact extensively with other
- Grundfos Scala 2 Recall
- Neptune Systems Trident
- Cocobay Antigua All Inclusive
- Marsha Formal Satin Tie Back Dress
- Affordable Travel Destinations
- Closeout Craft Supplies
- Warped Smiley Face Necklace
- Moschino Bubblegum Perfume 100ml
- Nike Blazer Mid 77 Jumbo Glaze Powder
- Air Force 1 '07 Premium Pineapple
- Digital Heat Fx White Toner Printer
- Sublimation Transfer Instructions
- Plinth Coffee Table Rose Marble
- Santa Fe Architectural Walking Tour
- 40 Inch Round Wood Coffee Table
- Primitive Americana Decor


